Smart Questions to Ask in a Parent-Teacher Meeting | PTM Made Easy
Parent-Teacher Meetings (PTMs) are more than quick updates on marks — they’re a chance to build a real partnership between home and school. A good Parent-Teacher Meeting conversation helps parents see beyond grades. It opens up insights about a child’s strengths, struggles, emotions and even hidden talents. When parents participate actively, they don’t just track […]
Read More
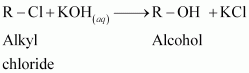



Comments
Basic medium does not mean RO-. There can be many sources of OH-. Poor explanation..
After searching in diff sites finally find a perfect answer to the question
Thanks for making me understand
Ghosh