Class 12 Chemistry - Chapter General Principles and Processes of Isolation of Elements NCERT Solutions | Write down the reactions taking place in
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
During the extraction of iron, the reduction of iron oxides takes place in the blast furnace. In this process, hot air is blown from the bottom of the furnace and coke is burnt to raise the temperature up to 2200 K in the lower portion itself. The temperature is lower in the upper part. Thus, it is the lower part where the reduction of iron oxides (Fe2O3 and Fe3O4) takes place.
The reactions taking place in the lower temperature range (500 - 800 K) in the blast furnace are:
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2Fe3 + CO2
The reactions taking place in the higher temperature range (900 - 1500 K) in the blast furnace are:
C +CO2 → 2CO
FeO + CO → Fe + CO2
The silicate impurity of the ore is removed as slag by calcium oxide (CaO), which is formed by the decomposition of limestone (CaCO3).
CaCO3 → CaO + Co2
CaO + SiO2 → CaSiO3
Calcium silicate (Slag)

More Questions From Class 12 Chemistry - Chapter General Principles and Processes of Isolation of Elements
- Q:-
What is the significance of leaching in the extraction of aluminium?
- Q:-
Copper can be extracted by hydrometallurgy but not zinc. Explain.
- Q:-
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
- Q:-
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
- Q:-
The reaction,
Cr2O3 + 2Al → Al2O3 + 2Cr (ΔGo = -421kJ)
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
- Q:-
How can you separate alumina from silica in bauxite ore associated with silica? Give equations, if any.
- Q:-
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
- Q:-
Write chemical reactions taking place in the extraction of zinc from zinc blende.
- Q:-
Describe a method for refining nickel.
- Q:-
Why copper matte is put in silica lined converter?
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Why do soaps not work in hard water?
- Q:-
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
- Q:-
Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
- Q:-
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
- Q:-
Complete each synthesis by giving missing starting material, reagent or products
(i)
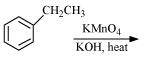
(ii)

(iii)

(iv)

(v)

(vi)
- Q:-
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
- Q:-
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
- Q:-
Write the structures of products of the following reactions;
(i)

(ii)

(iii)

(iv)

- Q:-
Predict the products of the following reactions:
(i)

(ii)

(iii)

(iv)

- Q:-
Give the IUPAC names of the following compounds:
(i) PhCH2CH2COOH (ii) (CH3)2C=CHCOOH
(iii)
 (iv)
(iv) 
4 Comment(s) on this Question
Awsm answer which I have saeen
Nice description!!!!
Best anwser than other meritation
this is a good answer
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
