Class 12 Chemistry - Chapter General Principles and Processes of Isolation of Elements NCERT Solutions | Copper can be extracted by hydrometallur
Copper can be extracted by hydrometallurgy but not zinc. Explain.
The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to displace copper from their solution.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
But to displace zinc, more reactive metals i.e., metals having lower reduction potentials than zinc such as Mg, Ca, K, etc. are required. But all these metals react with water with the evolution of H2 gas.
2K(s) + 2H2O (l) → 2KOH (aq) + H2(g)
As a result, these metals cannot be used in hydrometallurgy to extract zinc.
Hence, copper can be extracted by hydrometallurgy but not zinc.
More Questions From Class 12 Chemistry - Chapter General Principles and Processes of Isolation of Elements
- Q:-
What is the significance of leaching in the extraction of aluminium?
- Q:-
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
- Q:-
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
- Q:-
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
- Q:-
The reaction,
Cr2O3 + 2Al → Al2O3 + 2Cr (ΔGo = -421kJ)
is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
- Q:-
How can you separate alumina from silica in bauxite ore associated with silica? Give equations, if any.
- Q:-
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
- Q:-
Write chemical reactions taking place in the extraction of zinc from zinc blende.
- Q:-
Describe a method for refining nickel.
- Q:-
Why copper matte is put in silica lined converter?
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Why do soaps not work in hard water?
- Q:-
Arrange the following compounds in increasing order of their boiling points.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
- Q:-
Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
- Q:-
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
- Q:-
Complete each synthesis by giving missing starting material, reagent or products
(i)
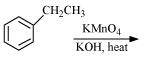
(ii)

(iii)

(iv)

(v)

(vi)
- Q:-
Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
- Q:-
Calculate the osmotic pressure in pascals exerted by a solution prepared by dissolving 1.0 g of polymer of molar mass 185,000 in 450 mL of water at 37°C.
- Q:-
Write the structures of products of the following reactions;
(i)

(ii)

(iii)

(iv)

- Q:-
Predict the products of the following reactions:
(i)

(ii)

(iii)

(iv)

- Q:-
Give the IUPAC names of the following compounds:
(i) PhCH2CH2COOH (ii) (CH3)2C=CHCOOH
(iii)
 (iv)
(iv) 
7 Comment(s) on this Question
Nice
nycð¥°ð¥°â©à¼¼Ëµâ¯â¿â¯Ëµà¼½ã¤Â¤=[]:::::>(â¿ ââ¿â) áââ°Uâ¯
Appropriate â
Nyc one and helpful for me
helpful! thanks a lot
Thnq
helpful info
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
