Class 12 Chemistry - Chapter Coordination Compounds NCERT Solutions | Discuss the nature of bonding in the fol
Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3-
(iv) [CoF6]3-
(i) [Fe(CN)6]4-
In the above coordination complex, iron exists in the +II oxidation state.
Fe2+ : Electronic configuration is 3d6
Orbitals of Fe2+ ion:

Hence, the geometry of the complex is octahedral and the complex is diamagnetic (as there are no unpaired electrons).
(ii) [FeF6]3-
In this complex, the oxidation state of Fe is +3.
Orbitals of Fe+3 ion:

Hence, the geometry of the complex is found to be octahedral.
(iii) [Co(C2O4)3]3-
Cobalt exists in the +3 oxidation state in the given complex.
Orbitals of Co3+ ion:

Hence, the geometry of the complex is found to be octahedral.
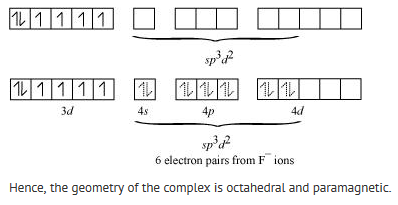
(iv) [CoF6]3- Cobalt exists in the +3 oxidation state.
Orbitals of Co3+ ion:

Again, fluoride ion is a weak field ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridization. sp3d2 hybridized orbitals of Co3+ ion are:
More Questions From Class 12 Chemistry - Chapter Coordination Compounds
- Q:-
[NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why?
- Q:-
Explain on the basis of valence bond theory that [Ni(CN)4]2- ion with square planar structure is diamagnetic and the [Ni(CN)4]2- ion with tetrahedral geometry is paramagnetic.
- Q:-
Draw figure to show the splitting of d orbitals in an octahedral crystal field.
- Q:-
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O
(ii) [Co(NH3)5Cl]Cl2
(iii) CrCl3(py)3
(iv) Cs[FeCl4]
(v) K4[Mn(CN)6]
- Q:-
The oxidation number of cobalt in K[Co(CO)4] is
(i) +1
(ii) +3
(iii) -1
(iv) -3
- Q:-
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2- is diamagnetic. Explain why?
- Q:-
Discuss the nature of bonding in metal carbonyls.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2- ion.
- Q:-
Using IUPAC norms write the formulas for the following:
(i) Tetrahydroxozincate(II)
(ii) Potassium tetrachloridopalladate(II)
(iii) Diamminedichloridoplatinum(II)
(iv) Potassium tetracyanonickelate(II)
(v) Pentaamminenitrito-O-cobalt(III)
(vi) Hexaamminecobalt(III) sulphate
(vii) Potassium tri(oxalato)chromate(III)
(viii) Hexaammineplatinum(IV)
(ix) Tetrabromidocuprate(II)
(x) Pentaamminenitrito-N-cobalt(III)
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Henry's law constant for CO2 in water is 1.67 x 108Pa at 298 K. Calculate the quantity of CO2in 500 mL of soda water when packed under 2.5 atm CO2 pressure at 298 K.
- Q:-
Sea is the greatest source of some halogens. Comment.
- Q:-
How are the following conversions carried out?
(i) Propene → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
Time required to decompose SO2Cl2to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
- Q:-
Complete the following reactions:
(i) C2H4 + O2 →
(ii) 4Al + 3O2 →
- Q:-
Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
- Q:-
Explain why fluorine forms only one oxoacid, HOF.
- Q:-
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3with platinum electrodes.
(iii) A dilute solution of H2SO4with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
- Q:-
Distinguish between
(i)Hexagonal and monoclinic unit cells
(ii) Face-centred and end-centred unit cells.
5 Comment(s) on this Question
Last-minute helppp.. really appreciateable.....!
Its nice,congratulations saralstudy
It is really helpful
Make something original
Most Useful page for a Indian Student.
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
