Class 12 Chemistry - Chapter Chemical Kinetics NCERT Solutions | The rate constant for the decomposition
The rate constant for the decomposition of N2O5 at various temperatures is given below:
|
T/°C |
0 | 20 | 40 | 60 | 80 |
|
105 X K /S-1 |
0.0787 | 1.70 | 25.7 | 178 | 2140 |
Draw a graph between ln k and 1/T and calculate the values of A and Ea.
Predict the rate constant at 30 º and 50 ºC.
From the given data, we obtain
| T/°C | 0 | 20 | 40 | 60 | 80 |
|
T/K |
273 | 293 | 313 | 333 | 353 |
| 1/T / k-1 |
3.66×10 - 3 |
3.41×10 - 3 |
3.19×10 - 3 |
3.0×10 - 3 |
2.83 ×10 - 3 |
| 105 X K /S-1 | 0.0787 | 1.70 | 25.7 | 178 | 2140 |
| In k | -7.147 | -4.075 | -1.359 | -0.577 | 3.063 |
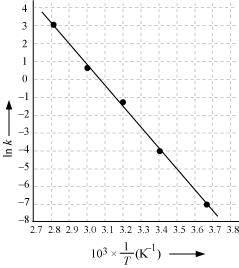
Slope of the line,

In k= - 2.8
Therefore, k = 6.08x10-2s-1
Again when T = 50 + 273K = 323K,
1/T = 3.1 x 10-3 K
In k = - 0.5
Therefore, k = 0.607 s-1
More Questions From Class 12 Chemistry - Chapter Chemical Kinetics
- Q:-
The half-life for radioactive decay of 14C is 5730 years. An archaeological artifact containing wood had only 80% of the 14C found in a living tree. Estimate the age of the sample.
- Q:-
For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
- Q:-
The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
- Q:-
A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
- Q:-
In a reaction, 2A → Products, the concentration of A decreases from 0.5 mol L-1 to 0.4 mol L-1 in 10 minutes. Calculate the rate during this interval?
- Q:-
The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y?
- Q:-
During nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If 1μg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically.
- Q:-
Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law, with t1/2 = 3.00 hours. What fraction of sample of sucrose remains after 8 hours?
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
The decomposition of A into product has value of k as 4.5 x 103 s-1 at 10°C and energy of activation 60 kJ mol-1. At what temperature would k be 1.5 x 104 s-1?
Popular Questions of Class 12 Chemistry
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
List the uses of Neon and argon gases.
- Q:-
The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 Kif the Henry's law constants for oxygen and nitrogen are 3.30 x 107 mm and 6.51 x 107mm respectively, calculate the composition of these gases in water.
- Q:-
Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water?
(i) phenol (ii) toluene (iii) formic acid (iv) ethylene glycol (v) chloroform (vi) pentanol.
- Q:-
Why are halogens strong oxidising agents?
- Q:-
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
- Q:-
Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water. Ka = 1.4 x 10-3, Kf = 1.86 K kg mol-1.
- Q:-
Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
- Q:-
Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
- Q:-
How is ammonia manufactured industrially?
- Q:-
Write chemical reactions taking place in the extraction of zinc from zinc blende.
1 Comment(s) on this Question
Gud
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
