Class 12 Chemistry - Chapter Aldehydes Ketones and Carboxylic Acids NCERT Solutions | Give plausible explanation for each of t
Give plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethylcyclohexanone does not.
(ii) There are two -NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
(i) Cyclohexanones form cyanohydrins according to the following equation.

In this case, the nucleophile CN - can easily attack without any steric hindrance. However, in the case of 2, 2, 6 trimethylcydohexanone, methyl groups at α-positions offer steric hindrances and as a result, CN - cannot attack effectively.

For this reason, it does not form a cyanohydrin.
(ii) Semicarbazide undergoes resonance involving only one of the two - NH2 groups, which is attached directly to the carbonyl-carbon atom.

Therefore, the electron density on - NH2 group involved in the resonance also decreases. As a result, it cannot act as a nucleophile. Since the other - NH2 group is not involved in resonance; it can act as a nucleophile and can attack carbonyl-carbon atoms of aldehydes and ketones to produce semicarbazones.
(iii) Ester along with water is formed reversibly from a carboxylic acid and an alcohol in presence of an acid.
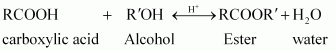
If either water or ester is not removed as soon as it is formed, then it reacts to give back the reactants as the reaction is reversible. Therefore, to shift the equilibrium in the forward direction i.e., to produce more ester, either of the two should be removed.
More Questions From Class 12 Chemistry - Chapter Aldehydes Ketones and Carboxylic Acids
- Q:-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
- Q:-
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal (ii) 2-Methylpentanal
(iii) Benzaldehyde (iv) Benzophenone
(v) Cyclohexanone (vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde (viii) Butan-1-ol
(ix) 2, 2-Dimethylbutanal
- Q:-
How will you convert ethanal into the following compounds?
(i) Butane-1, 3-diol (ii) But-2-enal (iii) But-2-enoic acid
- Q:-
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i)Ethanal, Propanal, Propanone, Butanone.
(ii)Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint:Consider steric effect and electronic effect.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
- Q:-
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
- Q:-
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii)CH2FCO2H or CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
(iv)

- Q:-
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii)Tollens' reagent
(iii) Semicarbazide and weak acid
(iv)Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
- Q:-
What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin (ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(vii) Imine
(ix) 2,4-DNP-derivative
(x) Schiff's base
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Write the equations for the preparation of 1-iodobutane from
(i) 1-butanol
(ii) 1-chlorobutane
(iii) but-1-ene.
- Q:-
Nalorphene (C19H21NO3), similar to morphine, is used to combat withdrawal symptoms in narcotic users. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 x 10-3m aqueous solution required for the above dose.
- Q:-
The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding between hydrogen and other elements of the group].
- Q:-
A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL-1, then what shall be the molarity of the solution?
- Q:-
Calculate the emf of the cell in which the following reaction takes place:
Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s)
Given that Eøcell = 1.05 V
- Q:-
Boiling point of water at 750 mm Hg is 99.63°C. How much sucrose is to be added to 500 g of water such that it boils at 100°C.Molal elevation constant for water is 0.52 K kg mol-1.
- Q:-
For the reaction:
2A + B → A2B
the rate = k[A][B]2with k= 2.0 x 10-6mol-2L2s-1. Calculate the initial rate of the reaction when [A] = 0.1 mol L-1, [B] = 0.2 mol L-1. Calculate the rate of reaction after [A] is reduced to 0.06 mol L-1.
- Q:-
How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten CaCl2.
(ii) 40.0 g of Al from molten Al2O3.
- Q:-
Can you store copper sulphate solutions in a zinc pot?
- Q:-
Write IUPAC names of the following compounds:


3 Comment(s) on this Question
Thank u so much for providing the answers
Thanks
Many conversions are wrong
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
