Class 12 Chemistry - Chapter Aldehydes Ketones and Carboxylic Acids NCERT Solutions | What is meant by the following terms? Gi
What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin (ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(vii) Imine
(ix) 2,4-DNP-derivative
(x) Schiff's base
(i) Cyanohydrin:
Cyanohydrins are organic compounds having the formula RR“²C(OH)CN, where R and R“² can be alkyl or aryl groups.

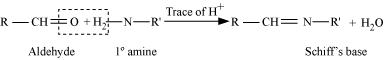
Aldehydes and ketones react with hydrogen cyanide (HCN) in the presence of excess sodium cyanide (NaCN) as a catalyst to field cyanohydrin. These reactions are known as cyanohydrin reactions.

Cyanohydrins are useful synthetic intermediates.
(ii) Acetal:
Acetals are gem - dialkoxy alkanes in which two alkoxy groups are present on the terminal carbon atom. One bond is connected to an alkyl group while the other is connected to a hydrogen atom.

When aldehydes are treated with two equivalents of a monohydric alcohol in the presence of dry HCl gas, hemiacetals are produced that further react with one more molecule of alcohol to yield acetal.

(iii) Semicarbarbazone:
Semicarbazones are derivatives of aldehydes and ketones produced by the condensation reaction between a ketone or aldehyde and semicarbazide.

Aldehydes and ketones react with hydrogen cyanide (HCN) in the presence of excess sodium cyanide (NaCN) a a catalyst to field cyanohydrin. these reactions are called cyanohydrin reactions. These are useful synthetic intermediates. Semicarbazones are useful for identification and characterization of aldehydes and ketones.
(iv) Aldol:
A Ã�²–hydroxy aldehyde or ketone is known as an aldol. It is produced by the condensation reaction of two molecules of the same or one molecule each of two different aldehydes or ketones in the presence of a base.

(v) Hemiacetal:
Hemiacetals are α - alkoxyalcohols
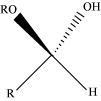
General structure of a hemiacetal
Aldehyde reacts with one molecule of a monohydric alcohol in the presence of dry HCl gas.

(vi) Oxime:
Oximes are a class of organic compounds having the general formula RR“²CNOH, where R is an organic side chain and R“² is either hydrogen or an organic side chain. If R“² is H, then it is known as aldoxime and if R“² is an organic side chain, it is known as ketoxime.

On treatment with hydroxylamine in a weakly acidic medium, aldehydes or ketones form oximes.

(vii) Ketal:
Ketals are gem - dialkoxyalkanes in which two alkoxy groups are present on the same carbon atom within the chain. The other two bonds of the carbon atom are connected to two alkyl groups.

Ketones react with ethylene glycol in the presence of dry HCl gas to give a cyclic product known as ethylene glycol ketals.

(viii) Imine:
Imines are chemical compounds containing a carbon nitrogen double bond.

Imines are produced when aldehydes and ketones react with ammonia and its derivatives.

(ix) 2, 4 - DNP - derivative:
2, 4 - dinitrophenylhydragones are 2, 4 - DNP - derivatives, which are produced when aldehydes or ketones react with 2, 4 - dinitrophenylhydrazine in a weakly acidic medium.

To identify and characterize aldehydes and ketones, 2, 4 - DNP derivatives are used.
(x) Schiff's base:
Schiff's base (or azomethine) is a chemical compound containing a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group-but not hydrogen. They have the general formula R1R2C = NR3. Hence, it is an imine.
It is named after a scientist, Hugo Schiff.
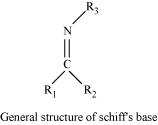
Aldehydes and ketones on treatment with primary aliphatic or aromatic amines in the presence of trace of an acid yields a Schiff's base.
More Questions From Class 12 Chemistry - Chapter Aldehydes Ketones and Carboxylic Acids
- Q:-
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
- Q:-
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal (ii) 2-Methylpentanal
(iii) Benzaldehyde (iv) Benzophenone
(v) Cyclohexanone (vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde (viii) Butan-1-ol
(ix) 2, 2-Dimethylbutanal
- Q:-
How will you convert ethanal into the following compounds?
(i) Butane-1, 3-diol (ii) But-2-enal (iii) But-2-enoic acid
- Q:-
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i)Ethanal, Propanal, Propanone, Butanone.
(ii)Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint:Consider steric effect and electronic effect.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
- Q:-
Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
- Q:-
Give plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6 trimethylcyclohexanone does not.
(ii) There are two -NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
- Q:-
Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii)CH2FCO2H or CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
(iv)

- Q:-
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii)Tollens' reagent
(iii) Semicarbazide and weak acid
(iv)Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
- Q:-
Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Name the members of the lanthanoid series which exhibit +4 oxidation state and those which exhibit +2 oxidation state. Try to correlate this type of behavior with the electronic configurations of these elements.
- Q:-
Use Hund's rule to derive the electronic configuration of Ce3+ ion and calculate its magnetic moment on the basis of 'spin-only' formula.
- Q:-
Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
- Q:-
Henry's law constant for the molality of methane in benzene at 298 Kis 4.27 x 105 mm Hg. Calculate the solubility of methane in benzene at 298 Kunder 760 mm Hg.
- Q:-
How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
- Q:-
Explain how does the -OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
- Q:-
The chemistry of the actinoid elements is not so smooth as that of the Lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
- Q:-
How can you separate alumina from silica in bauxite ore associated with silica? Give equations, if any.
- Q:-
What are biodegradable and non-biodegradable detergents? Give one example of each.
- Q:-
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase.
2 Comment(s) on this Question
Very nice and usefull
It is much much better for me
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
