Class 12 Chemistry - Chapter Alcohols Phenols and Ethers NCERT Solutions | Explain the following with an example.
Explain the following with an example.
(i) Kolbe's reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
(i) Kolbe's reaction:
When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium phenoxide when treated with carbon dioxide, followed by acidification, undergoes electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This reaction is known as Kolbe's reaction.
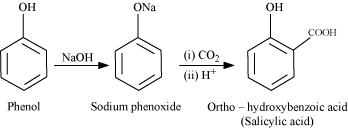
(ii) Reimer-Tiemann reaction:
When phenol is treated with chloroform (CHCl3) in the presence of sodium hydroxide, a -CHO group is introduced at the ortho position of the benzene ring.

This reaction is known as the Reimer-Tiemann reaction.
The intermediate is hydrolyzed in the presence of alkalis to produce salicyclaldehyde.

(iii) Williamson ether synthesis:
Williamson ether synthesis is a laboratory method to prepare symmetrical and unsymmetrical ethers by allowing alkyl halides to react with sodium alkoxides.

This reaction involves SN2 attack of the alkoxide ion on the alkyl halide. Better results are obtained in case of primary alkyl halides.
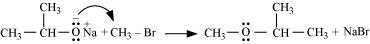
If the alkyl halide is secondary or tertiary, then elimination competes over substitution.
(iv) Unsymmetrical ether:
An unsymmetrical ether is an ether where two groups on the two sides of an oxygen atom differ (i.e., have an unequal number of carbon atoms). For example: ethyl methyl ether (CH3-O-CH2CH3).
More Questions From Class 12 Chemistry - Chapter Alcohols Phenols and Ethers
- Q:-
How are the following conversions carried out?
(i) Propene → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
- Q:-
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
- Q:-
Give the equations of reactions for the preparation of phenol from cumene.
- Q:-
Write equations of the following reactions:
(i) Friedel-Crafts reaction-alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft's acetylation of anisole.
- Q:-
Explain why is ortho nitrophenol more acidic than ortho methoxyphenol?
- Q:-
Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
- Q:-
Show how are the following alcohols prepared by the reaction of a suitable
Grignard reagent on methanal?
(i)

(ii)

- Q:-
Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
- Q:-
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
- Q:-
Give structures of the products you would expect when each of the following alcohol reacts with (a) HCl-ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol
(ii) 2-Methylbutan-2-ol
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Recently Viewed Questions of Class 12 Chemistry
- Q:-
Q1 : Write down the electronic configuration of:
(i) Cr3+ (iii) Cu+ (v) Co2+ (vii) Mn2+
(ii) Pm3+ (iv) Ce4+ (vi) Lu2+ (viii) Th4+
- Q:-
The decomposition of NH3on platinum surface is zero order reaction. What are the rates of production of N2and H2if k = 2.5 x 10-4mol-1L s-1?
- Q:-
How would you account for the following:
(i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidising.
(ii) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
(iii) The d1 configuration is very unstable in ions.
- Q:-
What is tincture of iodine ? What is its use ?
- Q:-
Explain [Co(NH3)6]3+ is an inner orbital complex whereas [Ni(NH3)6]2+ is an outer orbital complex.
- Q:-
Amongst the following, the most stable complex is
(i) [Fe(H2O)6]3+
(ii) [Fe(NH3)6]3+
(iii) [Fe(C2O4)3]3-
(iv) [FeCl6]3-
- Q:-
The activation energy for the reaction 2HI(g) → H2 + I2(g) is 209.5 kJ mol-1 at 581 K. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
What is the effect of temperature on the rate constant of a reaction? How can this temperature effect on rate constant be represented quantitatively?
- Q:-
What will be the effect of temperature on rate constant?
1 Comment(s) on this Question
V effective answers
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
