Class 12 Chemistry - Chapter Alcohols Phenols and Ethers NCERT Solutions | Explain why is ortho nitrophenol more ac
Explain why is ortho nitrophenol more acidic than ortho methoxyphenol?
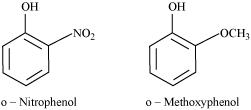
The nitro-group is an electron-withdrawing group. The presence of this group in the ortho position decreases the electron density in the O-H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
On the other hand, methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond and hence, the proton cannot be given out easily.
For this reason, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
More Questions From Class 12 Chemistry - Chapter Alcohols Phenols and Ethers
- Q:-
Explain the following with an example.
(i) Kolbe's reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
- Q:-
How are the following conversions carried out?
(i) Propene → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
- Q:-
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
- Q:-
Give the equations of reactions for the preparation of phenol from cumene.
- Q:-
Write equations of the following reactions:
(i) Friedel-Crafts reaction-alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft's acetylation of anisole.
- Q:-
Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
- Q:-
Show how are the following alcohols prepared by the reaction of a suitable
Grignard reagent on methanal?
(i)

(ii)

- Q:-
Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
- Q:-
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
- Q:-
Give structures of the products you would expect when each of the following alcohol reacts with (a) HCl-ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol
(ii) 2-Methylbutan-2-ol
Popular Questions of Class 12 Chemistry
- Q:-
For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02 M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
- Q:-
Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate(II)
(iii) Tris(ethane-1,2-diamine) chromium(III) chloride
(iv) Amminebromidochloridonitrito-N-platinate(II)
(v) Dichloridobis(ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron(III) hexacyanoferrate(II)
- Q:-
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
- Q:-
Why are solids rigid?
- Q:-
Write any two characteristics of Chemisorption.
- Q:-
Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde
(ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde
(iv) 4-Oxopentanal
(v) Di-sec-butyl ketone
(vi) 4-Fluoroacetophenone
- Q:-
Which of the ores mentioned in Table 6.1 can be concentrated by magnetic separation method?
- Q:-
Why are pentahalides more covalent than trihalides?
- Q:-
Silver atom has completely filled d orbitals (4d10) in its ground state. How can you say that it is a transition element?
- Q:-
Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Recently Viewed Questions of Class 12 Chemistry
- Q:-
What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
- Q:-
What are the different oxidation states exhibited by the lanthanoids?
- Q:-
Why are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide ?
- Q:-
What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements: 29, 59, 74, 95, 102, 104.
- Q:-
Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
- Q:-
Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
- Q:-
Why are Mn2+compounds more stable than Fe2+ towards oxidation to their +3 state?
- Q:-
What are artificial sweetening agents? Give two examples.
- Q:-
Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor, Why?
- Q:-
Explain why Cu+ ion is not stable in aqueous solutions?
10 Comment(s) on this Question
Great
thanks sir
Thanks
Thanksðð
nice answer but not perfectly shown
Very good answer
Very helpful
Thnkuuuuuuu it's very useful
Perfect answer ...thanx
Thanks its a great help to me
- All Chapters Of Class 12 Chemistry
- All Subjects Of Class 12
